-
Basic Knowledge of Steam
-
Steam Attributes
-
-
-
Water phases diagram
Figure 1.1 shows a graphical representation of the phases of water under atmospheric pressure. It illustrates the contents described in “Water phases”. The horizontal axis shows the specific enthalpy, and the vertical axis shows the temperature. The phase diagram shows the change of temperature and phase depending on the specific enthalpy contained in the water molecules.
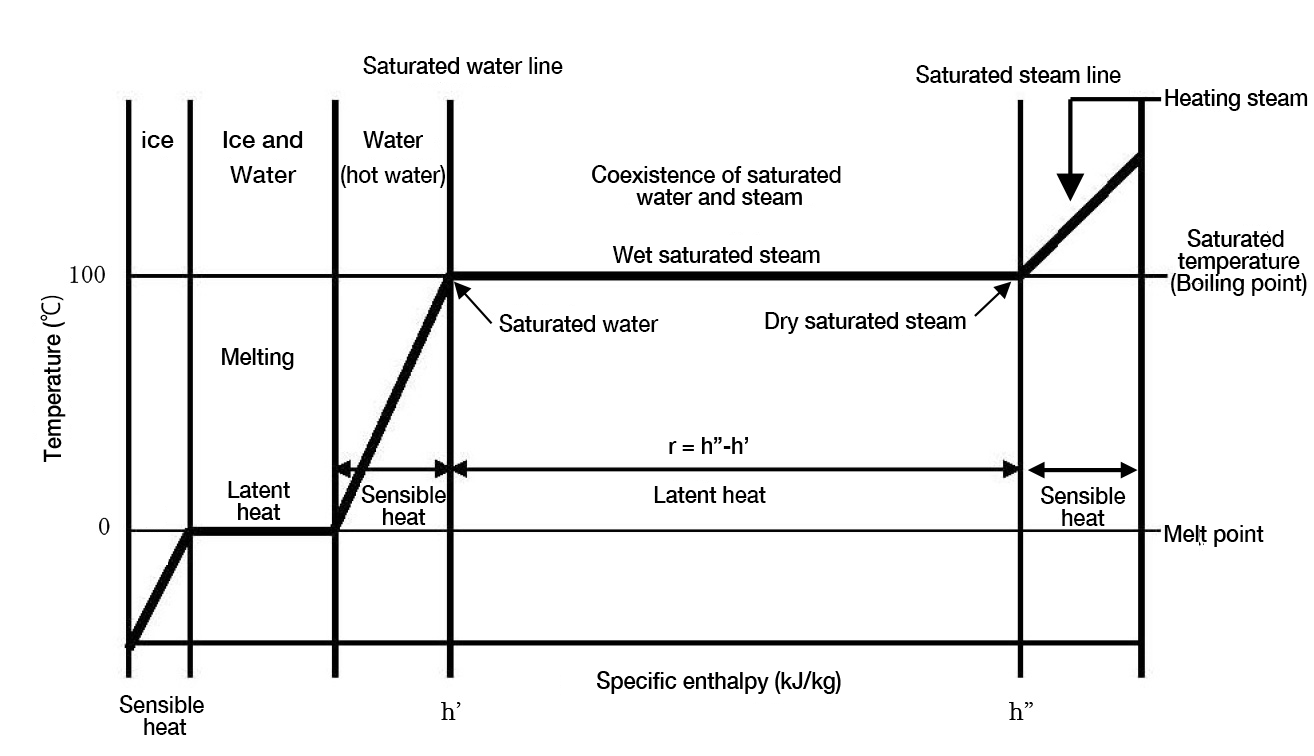
Figure1.1 Water phase diagram under atmospheric pressure
The above figure expresses the following items: sensible heat (enthalpy of saturated temperature, h’) before the water heats from 0 ˚C to 100 ˚C, total heat quantity when all saturated water becomes steam (enthalpy h” of saturated steam), and necessary latent heat for evaporation (enthalpy of evaporation r = h” – h’). The vapor in the state in which saturated water is evaporating is called wet saturated steam and where the steam has all evaporated, this is called dry saturated steam. As dry saturated steam is heated again, the temperature rises. Steam at a temperature much higher than saturated temperature is called superheated steam, and the temperature difference between the superheated steam and saturated steam is called superheat.
Hereafter, enthalpy of water is referred to as 'sensible heat', the enthalpy of evaporation as "latent heat," and the heat possessed by steam as 'latent heat' and the and the heat of vapor is'total heat'
-
The energy of steam
As we have mentioned, steam changes phase to become condensate after it is used for heating and loses its latent. The temperature at that point is the same as steam. Latent heat with this property is extremely effective energy in processes that require stable heat treatment at a constant temperature, sterilization, etc. The reason why steam is excellent as a carrier of energy is that it is a common substance that can hold a very large amount of latent heat.
You can confirm the amount of latent heat contained in a saturated steam table. Table 1.2 is a data excerpt from a saturated steam table. For instance, in the case of atmospheric pressure (gauge pressure 0.0 MPa), the contents are as follows (rounded to one decimal place).Sensible heat of saturated water :h’=419 kJ/kg
Total heat of steam : h”=2,676 kJ/kg
Latent heat : r=h”-h’=2,257 kJ/kgThus,
the proportion of total heat of steam) =2,257/2,676=0.8434≒84%
(the amount of latent heat retained by steam relative to sensible heat) =2,257/419=5.3866≒5.39
In this way, steam under atmospheric pressure has 84% of total heat as latent heat, or, put another way, its latent heat is 5.39 times the sensible heat.
Table 1.1 shows a comparison of thermodynamic properties of water, ammonia, methanol and ethanol. It can be seen that water has a comparatively large ratio of latent heat.
Table 1.1 Thermodynamic properties of common substances under atmospheric pressure
Substance
Melting point(℃)
Boiling point(℃)
Melting heat (kJ/kg)
Latent heat(kJ/kg)
Water
0
100
333.5
2,257
Ammonia
-77.8
-33.4
338
1,371
Methanol
-97.7
64.7
99.2
1,190
Ethanol
-114.1
78.6
109
855
As the pressure increases, more heat power is needed to reach saturation, and the temperature also increases without phase change. In other words, both the sensible heat and the saturation temperature increase. Figure 1.2 shows this relationship as a saturated vapor pressure curve. On this curve, both water and steam can coexist at the same saturation temperature. Below the curve is water that has not yet reached the saturation temperature, and above the curve is superheated steam.
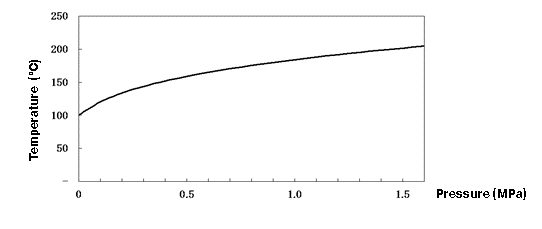
Figure 1.2 Saturated vapor pressure curve
How does the heat capacity of saturated steam and saturated water change as the pressure increase? Figure 1.3 is a diagram showing these relationships. The graph shows us the following facts.
1. Sensible heat of saturated water increases with the pressure of the water.
2. Latent heat of steam decreases as the pressure increases.
Total heat of steam (the sum of “1. sensible heat” and “2. latent heat”) is almost constant compared to its components, although it increases slightly in the low pressure range. (However, it conversely decreases from around 3.2MPa, and the latent heat becomes zero when the critical point is reached.)
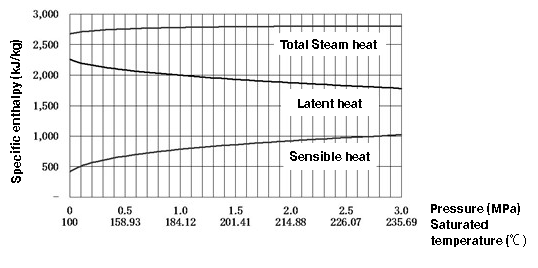
Figure 1.3 Relationship between heat quantity and pressure for steam and saturated water
What should be noted here is that the latent heat required for evaporation decreases with increasing pressure. This means that the higher the pressure of the steam, the less latent heat is available.. For instance, as show in Table 1.2 saturated steam table, the latent heat of 0.5 MPa and 1.0 MPa, r is respectively 2085 kJ/kg and 1,998kJ/kg. Thus, the latent heat of 1.0 MPa is lower than that of 0.5MPa and both are lower than 2,257kJ/kg, the latent heat under atmosphere pressure, (0.0 MPa).
Next, Figure 1.4 shows relations between the specific volume of steam and steam pressure. As the figure shows, the specific volume is inversely proportional, varying greatly In the low pressure range and decreasing as the pressure increases. The higher the pressure, the lower the latent heat per unit mass (1 kg), and also the lower its volume, resulting in an increase in latent heat per unit volume (1 m3). Therefore, by increasing the steam pressure, it is possible to carry more energy in a steam main line of relatively small size. This is one of the important points that should be considered when designing steam piping systems.
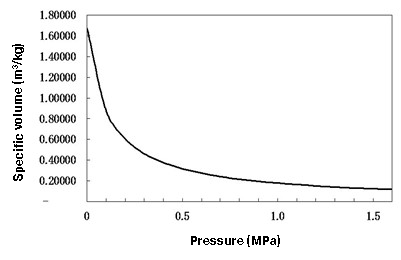
Figure1.4 Relationship between steam pressure and specific volume
Table 1.2 Saturated steam table (Gauge pressure basis)
Gauge pressure(MPa)
Saturated temperature(℃)
Specific volume (m3/kg)
Mass volume ratio (kg/m3)
Latent heat(kJ/kg)
Saturated water
Saturated steam
Sensible heat
Total
Latent heat
v’
v”
h’
h”
r=h”-h’
0.0
100.00
0.0010437
1.67300
0.5977
419.06
2676.0
2256.9
0.1
120.44
0.0010610
0.87999
1.1364
505.58
2706.6
2201.0
0.5
158.93
0.0011096
0.31482
3.1764
670.79
2755.6
2084.7
1.0
184.12
0.0011331
0.17718
5.6440
781.36
2779.7
1998.3
-
Dryness fraction
Steam is generated in an industrial boiler, and then transported to any location where it is used. Producing 100% dry steam in the industrial boiler is almost impossible, so industrial steam always contains a few droplets of water. Steam containing liquid water is called wet steam. However, 100% dry steam is required for most steam applications. The part of the wet steam that is actually steam instead of water is called ‘dryness fraction’ or just ‘dryness’. The higher the dryness fraction, the greater the quality of the steam.
The dryness fraction (χ) is the mass ratio of the dry steam compared to the whole mass of the wet steam. For instance, the dryness fraction is 0.95 when steam contains 5% water. In addition, (1-χ) is called wet fraction. The steam dry fraction of a typical boiler outlet is usually from 0.95 to 0.98. The heat quantity (specific enthalpy, h) which wet saturated steam has can be described using the following formula with the symbols in figure 1.1.
h=(1-χ)h’+χh”=h’+χr
-
Flash steam
The term “flash steam” is generally used to describe steam coming from condensate receiver vents and open-ended condensate discharge lines after steam traps. Why is steam formed without adding heat? Flash steam occurs if water with comparatively high temperature is depressurized so much that the saturation temperature of the new water pressure is below the original water temperature.
As soon as the saturation temperature of the water drops below its actual temperature, some of the water starts to evaporate. The following is an example of condensate passing a steam trap. Primary side temperature is nearly always high enough to let flash steam occur.
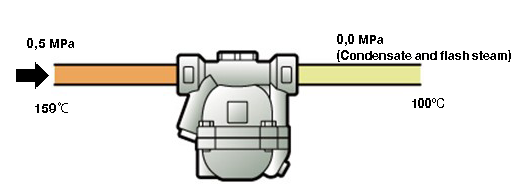
Figure 1.5 Flash steam generation
In figure 1.5, the primary side pressure of the steam trap is 0.5 MPa (which means a saturation temperature of about 159°C – the assumed water temperature for the example) and the condensate (assumed to be 1 kg for convenience in the following calculation) is discharged to a condensate line which is open to the atmosphere.
As shown in table 1.2, the sensible heat for 1kg condensate at 0.5 MPa saturated temperature is 671kJ. From the first law of thermodynamics, the total heat capacity of the fluid is equal between the high and low pressure sides of the steam trap, which generally follows the law of conservation of energy (heat loss due to heat dissipation and flow resistance in the steam trap is neglected). Therefore, 1 kg of water flowing to the low pressure side will have a heat capacity of 671 kJ. However, water at 0 MPa with saturation temperature (100°C) only has a sensible heat of 419 kJ, which means there is an imbalance of 671-419=252 kJ. From the perspective of water, this is surplus heat, but this surplus heat becomes latent heat and it boils some of the condensate, turning it into steam. That steam is called flash steam and the process is called flashing.
T Therefore, 1 kg of condensate, which exists as a liquid on the high pressure side of the trap, will exist as a liquid and partly as steam on the low pressure side.
The amount of created flash steam can be calculated using the following formula.
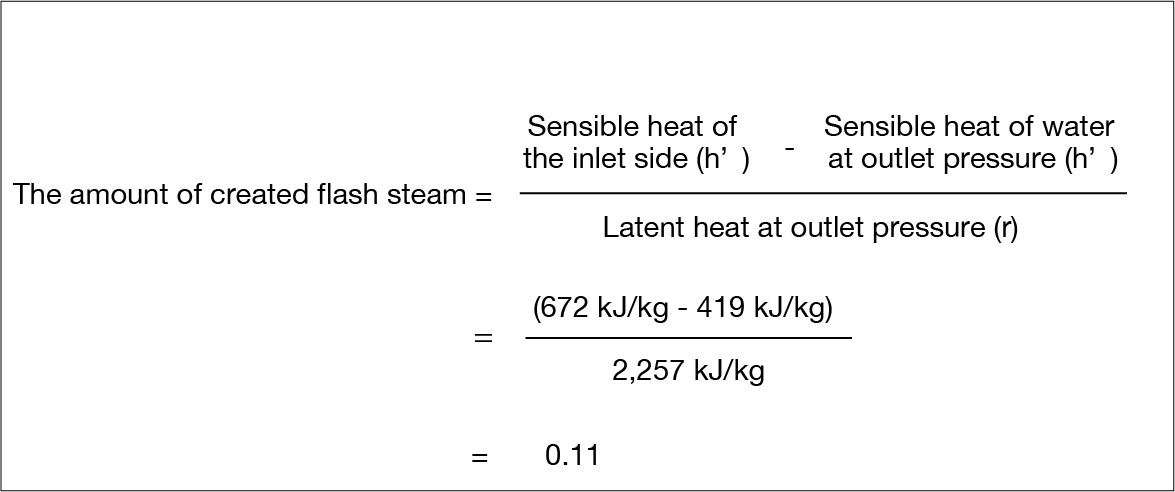
Thus, the amount of flash steam created per 1kg condensate is 0.11kg.
The fraction of flash steam generated upon depressurization is the ratio of surplus heat to the latent heat at the final pressure.
It is important to note that flash steam is not only a phenomenon that happens naturally in steam system but also an effect that is deliberately exploited and calculated to maximize the effectiveness of steam usage. A typical device for using flash vapor is a flash tank.
-
Other properties
As described above, the reason why steam is widely used as carrier of energy is that its relative amount of latent heat is large, and water is abundant on earth and is economical. The following points can also be mentioned.
- It is environmentally benign for humans.
- It is a non-corrosive, non- flammable, stable chemical.
- It has uniform heating compared to other heat source media.
- The saturated temperature can be controlled by changing the operating pressure. The required heating temperature can thus be provided by adjusting the pressure.
- Since the difference in specific volume between steam and condensate is large, new steam is supplied as soon as the steam condenses.
The following points are the problems of using steam.
- Unless a steam system is designed and maintained with the specific goal to keep it air free (which is a lot of effort), the mixing of air and (if applicable) other gases with the steam is inevitable. Such a mix reduces the heat-transfer efficiency significantly.
- The water used for steam generation is never pure. It always contains various impurities, which can cause various problems to the steam generation or the steam quality. Since the raw water used to generate steam is not pure water, impurities that cause oxidation and corrosion are dissolved in it, and these cannot be completely removed in the steam generation process.
-
Taking anti-freezing measures may be necessary depending on the steam trap location as the freezing point of water is 0°C